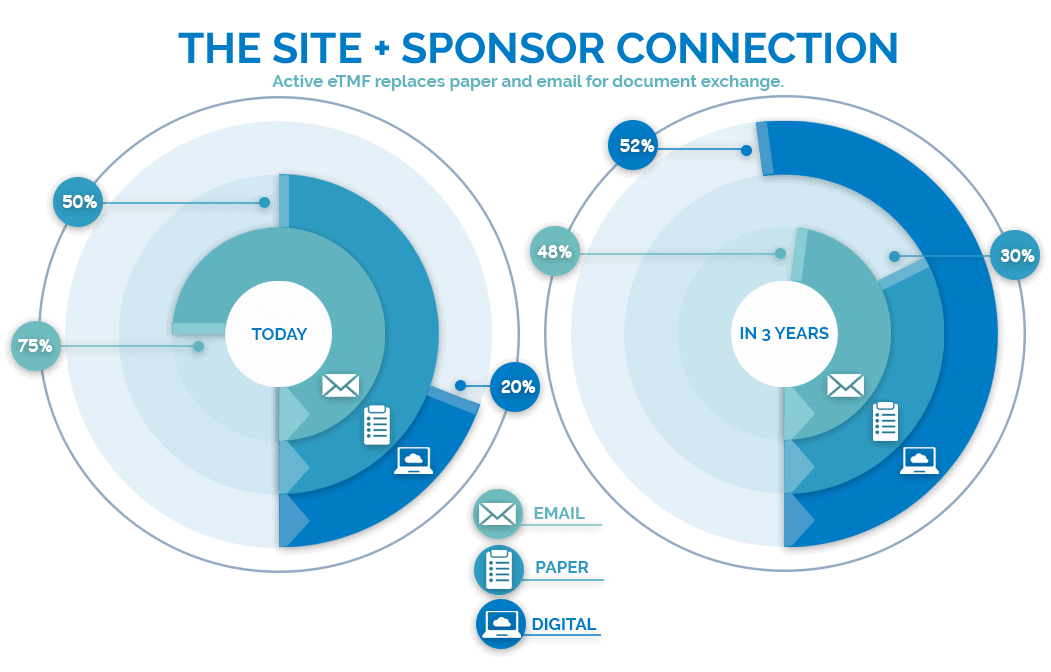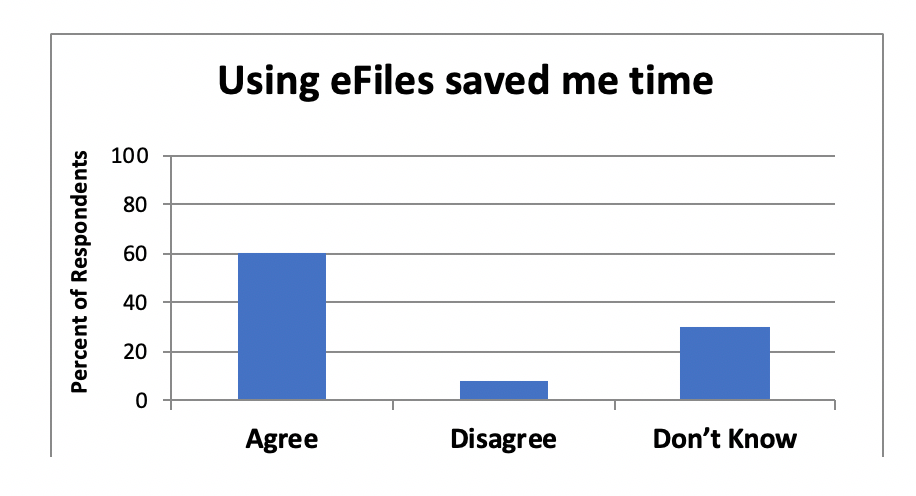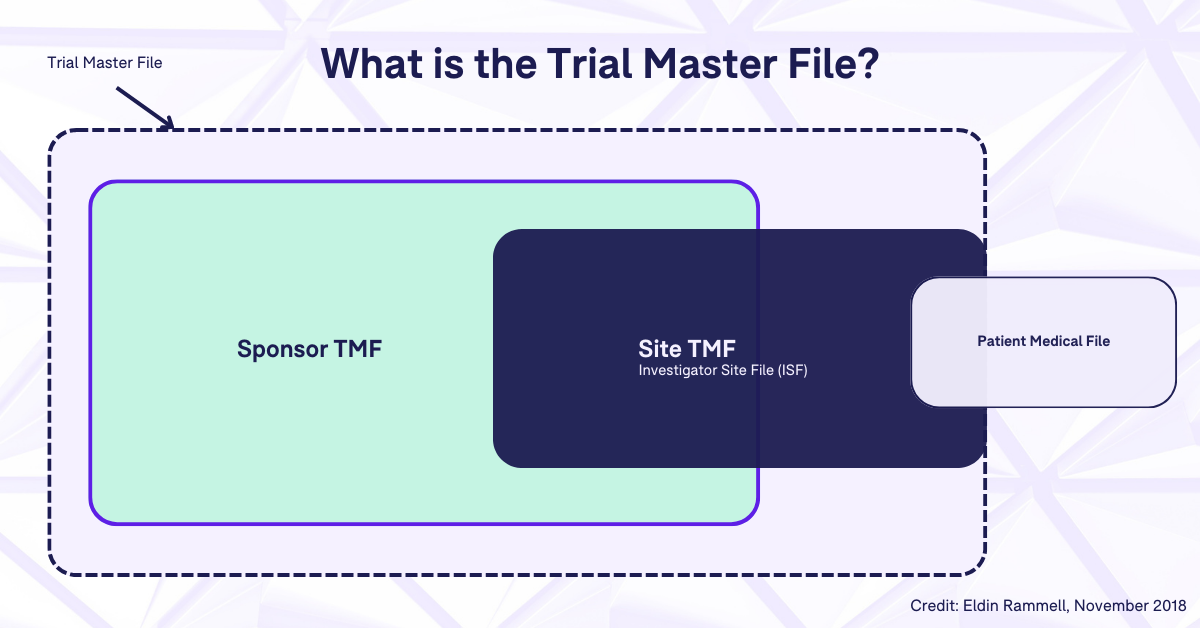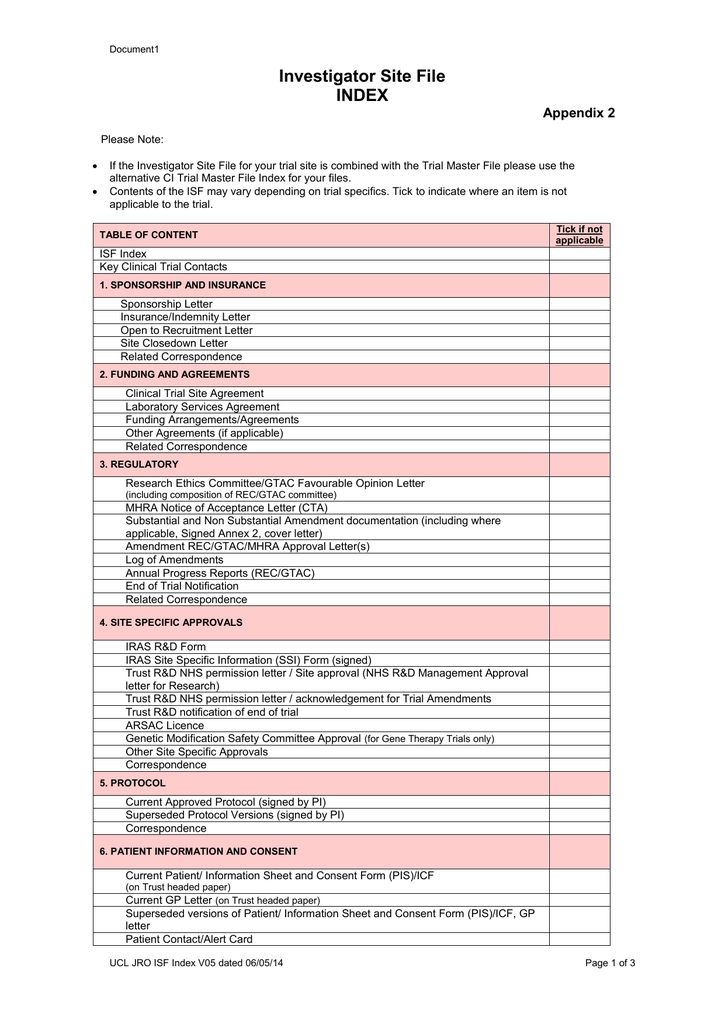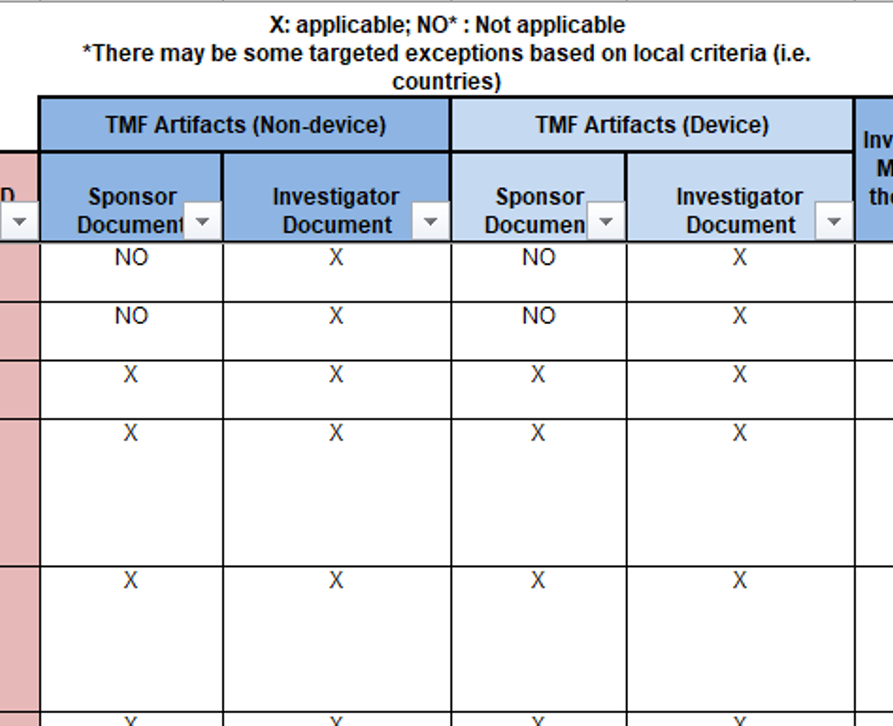
Is the TMF Reference Model applicable to the investigator site file / regulatory binder? – Trial Master File Reference Model
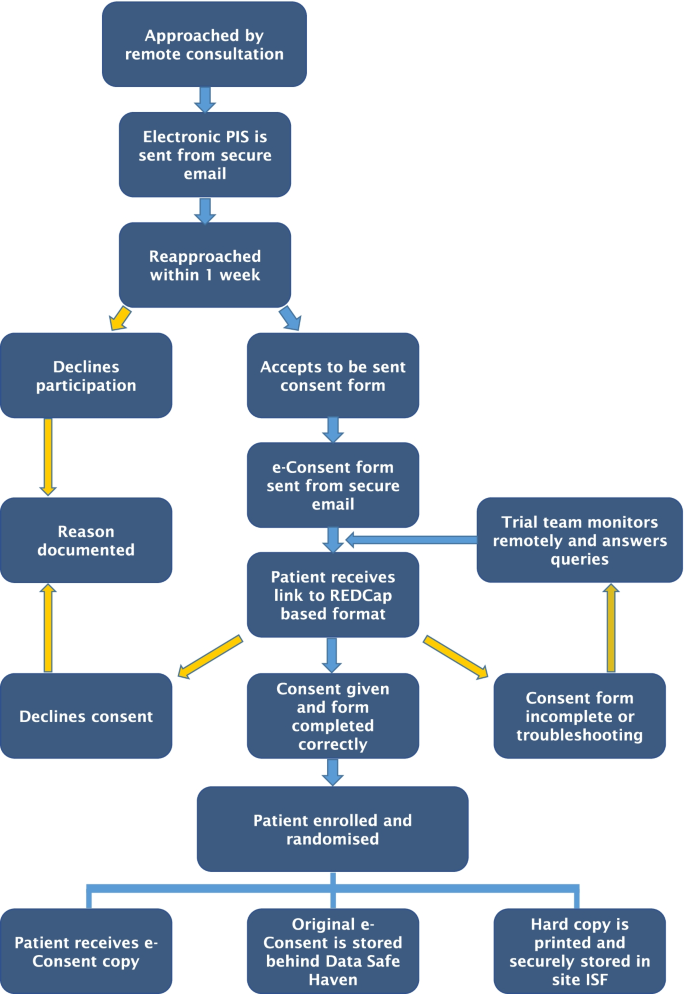
E-Consent—a guide to maintain recruitment in clinical trials during the COVID-19 pandemic | Trials | Full Text

Investigative Site Files and Trial Master Files Should Talk to Each Other - LMK Clinical Research, LLC