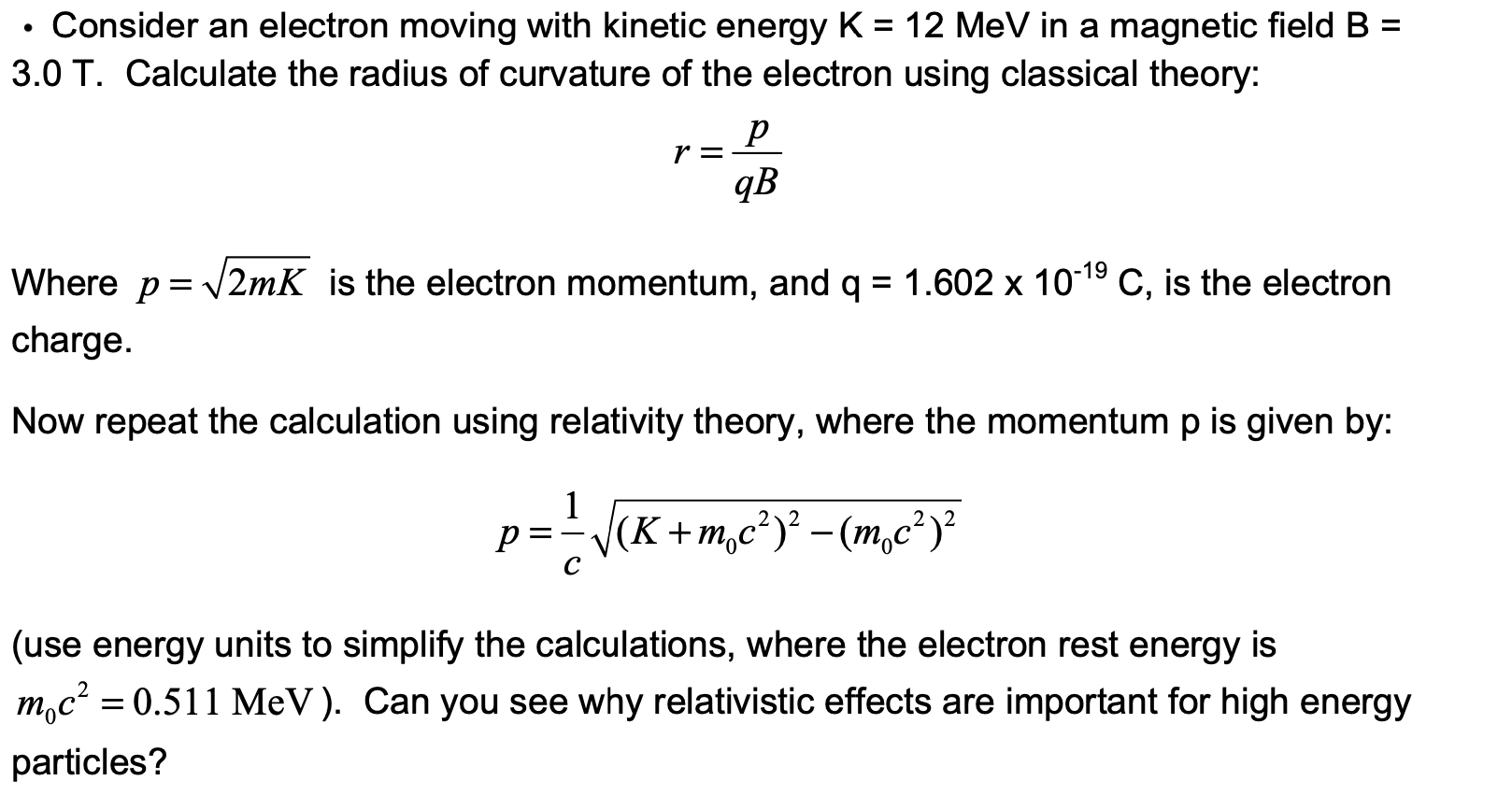
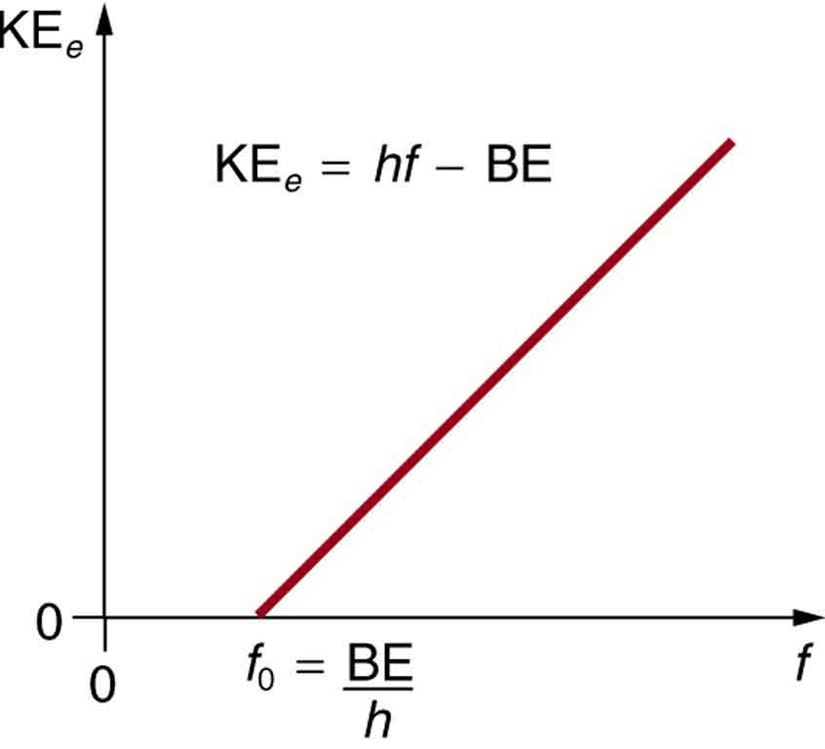
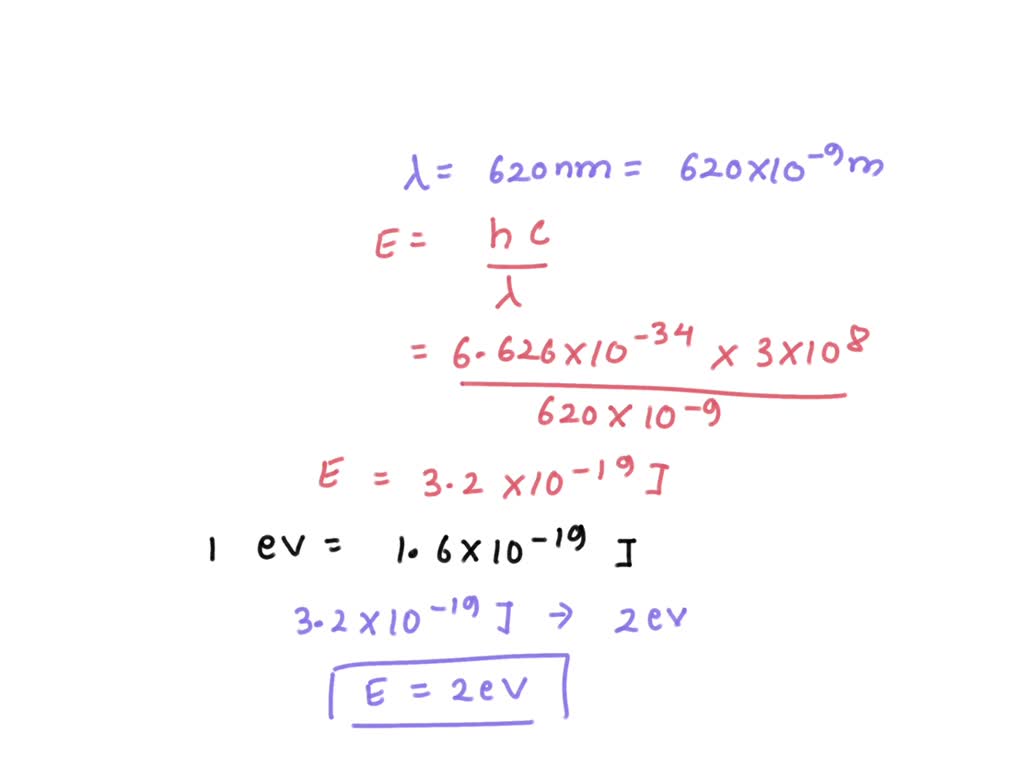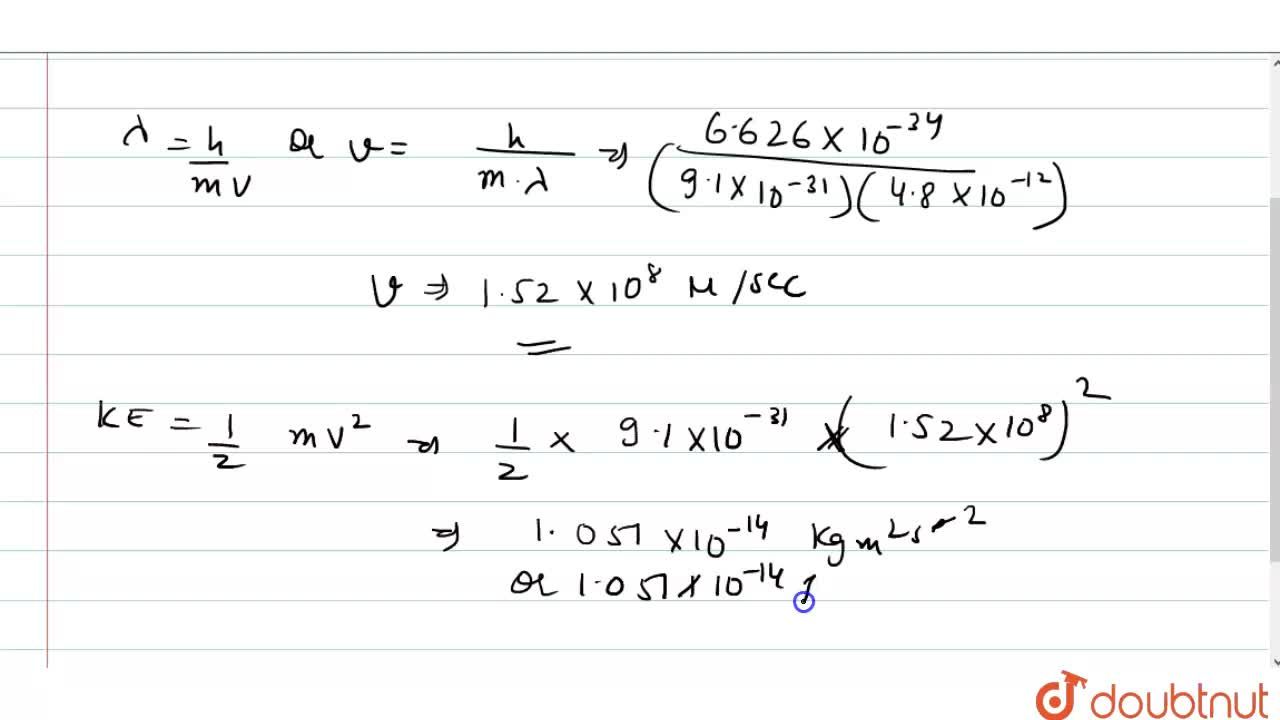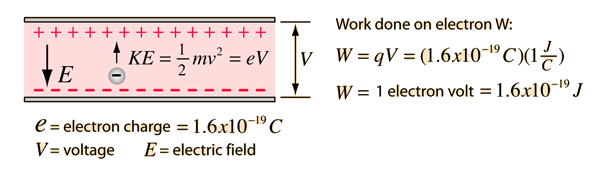kinetic energy of an electron which is associated with de Broglie's wavelength 20 angstrom is 1)1.0eV, 2)1.51eV, 3)0.59eV, 4)0.38eV.

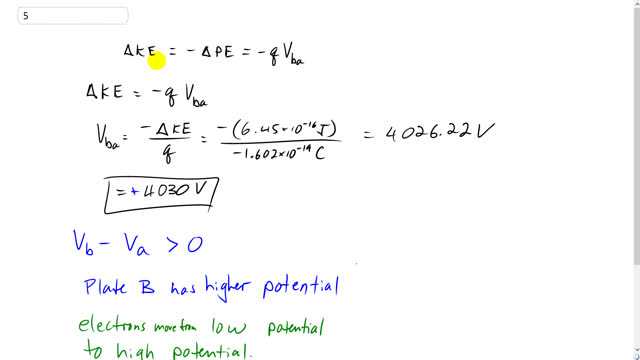
The ratio of kinetic energy and potential energy of an electron in a Bohr orbit of a hydrogen - like series is :
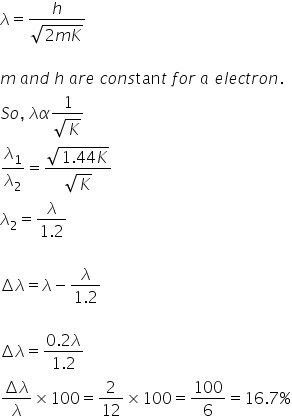
if the kinetic energy of electron increases by 44 find the percentage change in de brogile 39 s wavelength 0x1eny44 -Chemistry - TopperLearning.com

Question Video: Calculating the Kinetic Energy of an Electron Moving through a Negative Potential Difference | Nagwa

An electron is moving with a kinetic energy of 2.275 X ${{10}^{-25}}$ J. Calculate its de-Broglie wavelength. (Mass of electron = 9.1 x ${{10}^{-31}}$ kg, h = 6.6 x ${{10}^{-34}}$ Js - CBSE Class 11 Chemistry - Learn CBSE Forum

Total energy E, kinetic energy T , single electron energy E , Coulomb... | Download Scientific Diagram
The (average) kinetic energy of the free electrons (mass = m, |charged| = e), in a metal, at a temperature T in kelvin, equals - Sarthaks eConnect | Largest Online Education Community

homework and exercises - Is the kinetic energy of an electron always $1.6 \cdot 10^{-19}~\text{J}$? - Physics Stack Exchange
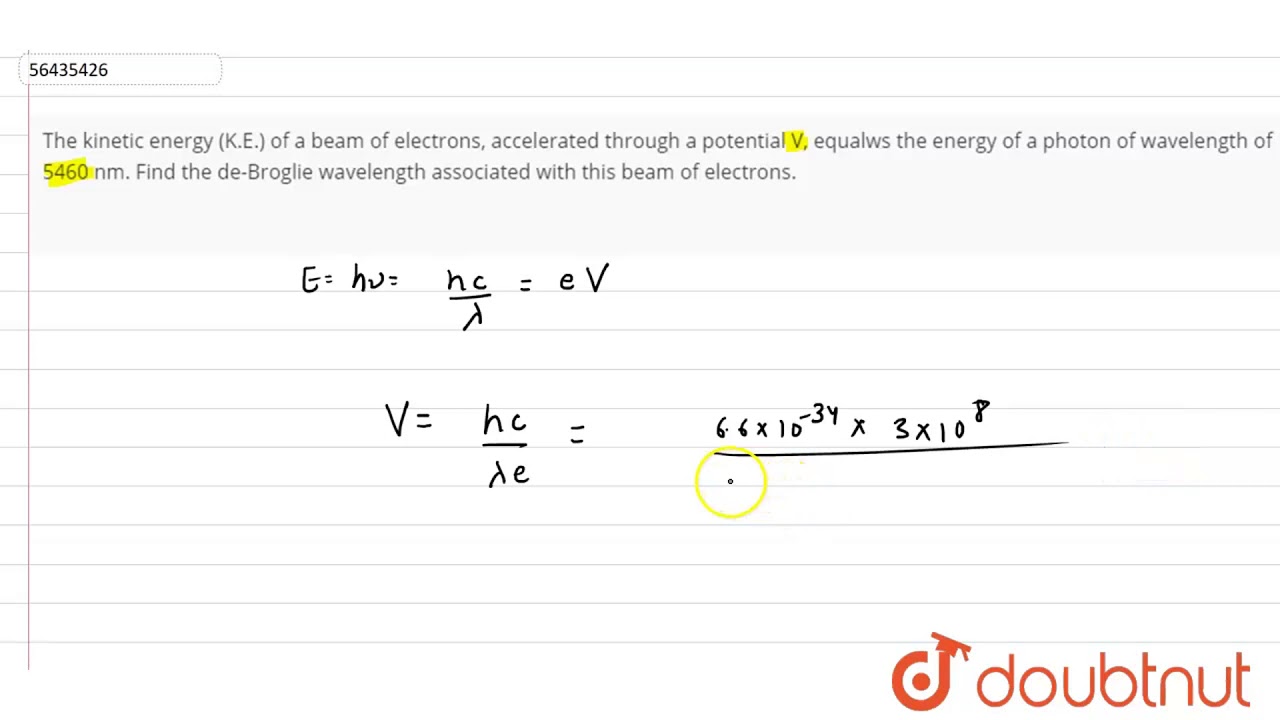
The kinetic energy (K.E.) of a beam of electrons, accelerated through a potential V, equalws - YouTube

Particle velocity as a function of kinetic energy for electron (black... | Download Scientific Diagram