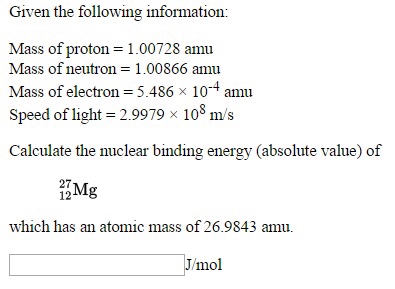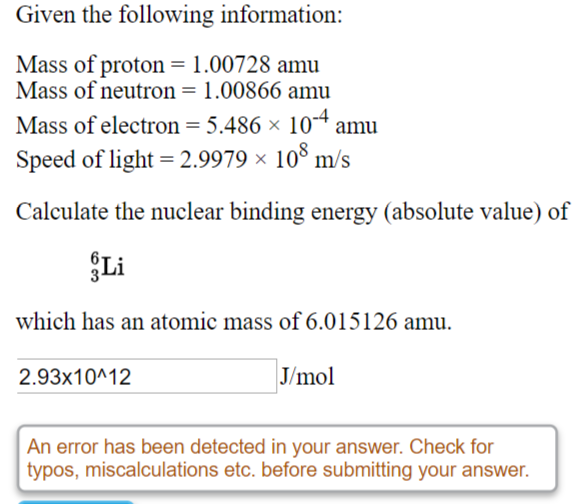
SOLVED: Put these in order from smallest t0 largest mass aiom 5 amu N3- ion 2 protons Multiple Choice Mkm<iv<| M<IV < I < | Iv <a € L4a<v IV <<< Erev 17 Bf 31 Nex Suvcd

Find the binding energy of `Na^(23)` . Atomic mass of `Na^(23)` is 22.9898 amu and that of ,,,,,, is - YouTube
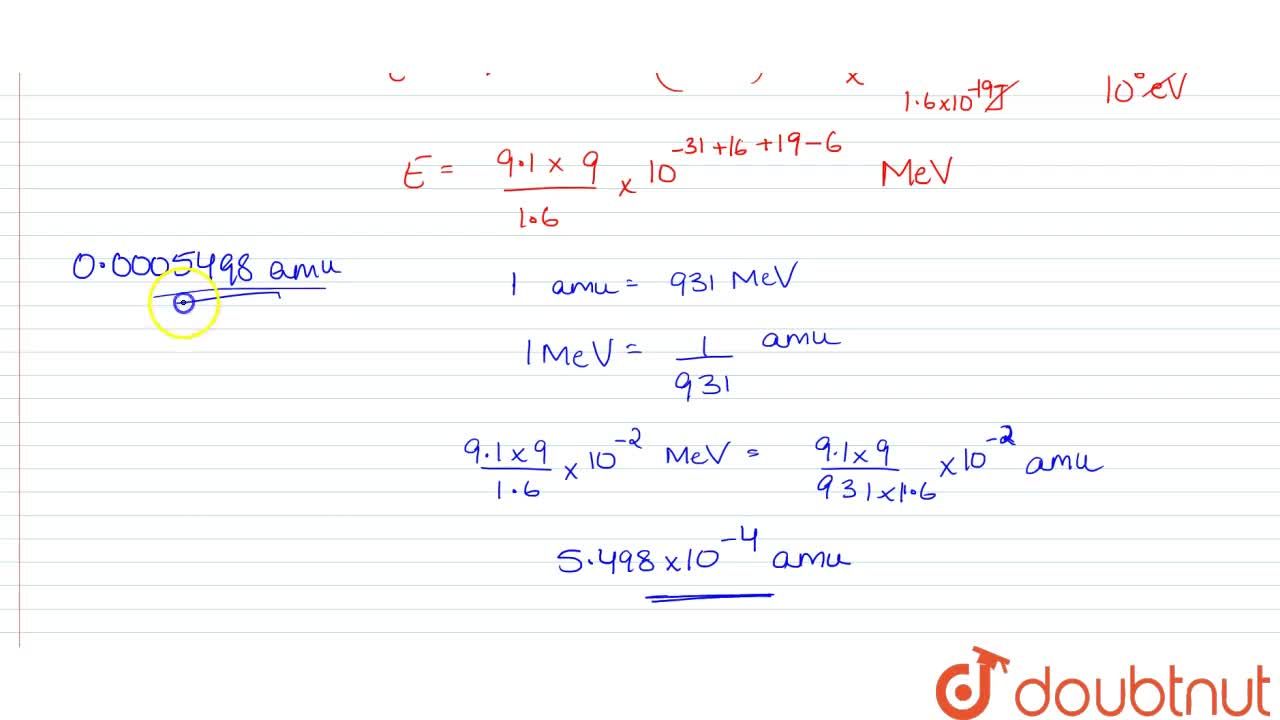
The mass of an electron can be expressed as (A) 0.512 MeV (B) 8.19 × 10–14J/c2 (C) 9.1 × 10–31 kg (D) 0.00055 amu
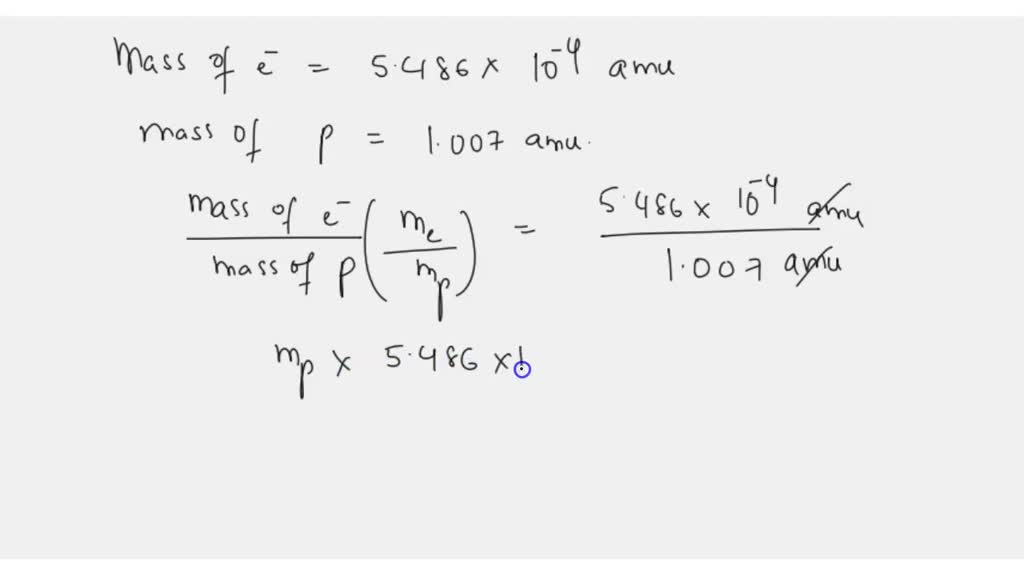
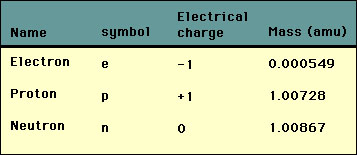
SOLVED: Given that the mass of an electron is 5.486 × 10^-4 amu, and the mass of a proton is 1.007 amu, calculate how many times heavier a proton is than an electron.

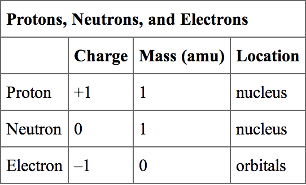
The masses of neutron and proton are 1.0087 and 1.0073 amu respectively. If the neutrons and protons combine to form helium nucleus of mass 4.0015 amu the binding energy of the helium
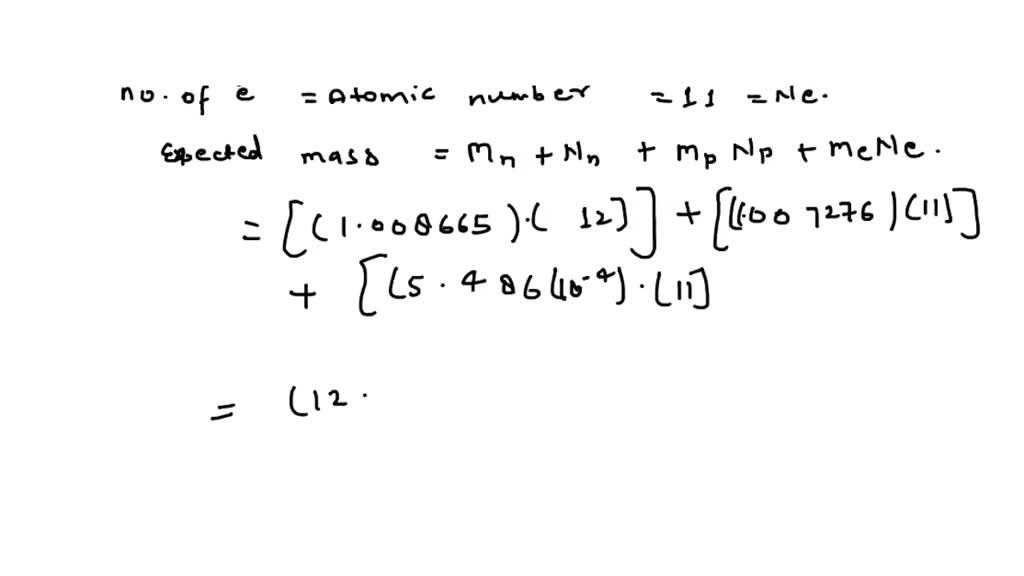
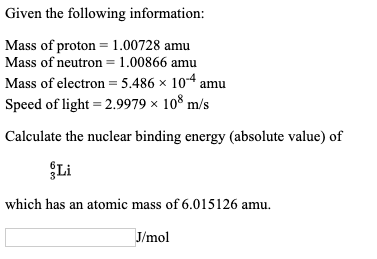
SOLVED: The proton mass is 1.007276 amu , the neutron mass is 1.008665 amu , and the electron mass is 5.486×10−4 amu . A. What is the expected mass of a sodium-23